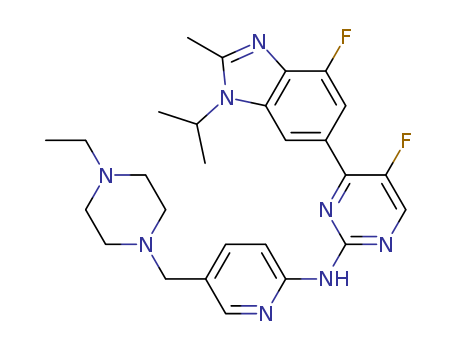
1231929-97-7
- Product Name:Abemaciclib
- Molecular Formula:C27H32F2N8
- Purity:99%
- Molecular Weight:506.601
Product Details:
CasNo: 1231929-97-7
Molecular Formula: C27H32F2N8
Quality Manufacturer Supply Best Quality Abemaciclib 1231929-97-7 Safe Transportation
- Molecular Formula:C27H32F2N8
- Molecular Weight:506.601
- Boiling Point:689.3±65.0 °C(Predicted)
- PKA:7.69±0.10(Predicted)
- PSA:75.00000
- Density:1.32±0.1 g/cm3(Predicted)
- LogP:4.88570
Abemaciclib(Cas 1231929-97-7) Usage
|
Description |
Abemaciclib, marketed under the brand name Verzenio™, is a medication developed by Eli Lilly and Company for the treatment of advanced or metastatic breast cancers. It is specifically used in the management of hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) breast cancer. Abemaciclib may be synthesized in a four step manner using a Suzuki coupling, followed by a Buchwald–Hartwig amination with the final step being a reductive amination using the Leuckart reaction. |
| Uses |
Advanced or Metastatic Breast Cancer: Abemaciclib has been approved in the USA for the treatment of HR+ HER2- advanced or metastatic breast cancer, both as a monotherapy and in combination with other therapies. a. In Combination with Fulvestrant: Used in women with disease progression following endocrine therapy. b. As Monotherapy: Administered in adult patients with disease progression following endocrine therapy and prior chemotherapy in the metastatic setting. Abemaciclib is also being investigated internationally for its potential efficacy in various other cancers beyond breast cancer. |
| Side Effects | Highest any-grade incidence of abemaciclib-associated AEs across trials include diarrhea (82-90%), fatigue (41-65%), nausea (30-64%) and neutropenia (37-50%). Hair thinning and hair loss are very common when taking abemaciclib with an aromatase inhibitor. Find out more about hair loss. This treatment is usually well tolerated but there are some possible side effects that you need to be aware of. The doctors, nurses and pharmacists can give you advice or answer any questions you may have. Diarrhoea is a very common side effect of Abemaciclib. This is usually worst in the first month of treatment. |
| Quality Manufacturer | Hangzhou Huarong Pharm Co., Ltd. is a quality manufacturer of Abemaciclib. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
1231929-97-7 Relevant articles
A synthesis of abemaciclib utilizing a Leuckart-Wallach reaction
Takeshi Kotake &Masakazu Toi
, Expert Opinion on Pharmacotherapy Volume 19, 2018 - Issue 5
Another problem is that its contribution to overall survival (OS) has not been shown. And while two large the phase 3 study highlighted the anti-tumour effect of abemaciclib, the OS results are awaited. Furthermore, the effect on brain metastases is expected to be unique to abemaciclib as the response of brain metastasis in HR-positive breast cancer patients has been confirmed in a few cases with case collection still ongoing.
Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors
Amita Patnaik; Lee S. Rosen; Sara M. Tolaney; Anthony W. Tolcher; Jonathan W. Goldman; Leena Gandhi; Kyriakos P. Papadopoulos; Muralidhar Beeram; Drew W. Rasco; John F. Hilton; Aejaz Nasir; Richard P. Beckmann; Andrew E. Schade; Angie D. Fulford; Tuan S. Nguyen; Ricardo Martinez; Palaniappan Kulanthaivel; Lily Q. Li; Martin Frenzel; Damien M. Cronier; Edward M. Chan; Keith T. Flaherty; Patrick Y. Wen; Geoffrey I. Shapiro
, Cancer discovery, 2016, Volume 6, Issue 7
Abemaciclib represents the first selective inhibitor of CDK4 and CDK6 with a safety profile allowing continuous dosing to achieve sustained target inhibition. This first-in-human experience demonstrates single-agent activity for patients with advanced breast cancer, NSCLC, and other solid tumors.
Abemaciclib: a CDK4/6 inhibitor for the treatment of HR+/HER2− advanced breast cancer
Silvia Paola Corona &Daniele Generali
Drug Design, Development and Therapy Volume 12, 2018 - Issue
Abemaciclib is the latest CDK4/6 inhibitor approved by the US Food and Drug Administration (FDA) in view of the results of the MONARCH 1 and 2 trials. Further trials are ongoing as other important questions await response.
1231929-97-7 Process route
-
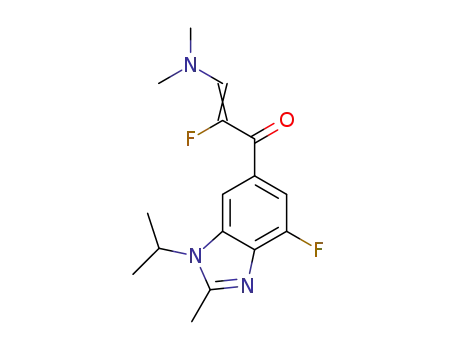
-
6-(3-N,N-dimethylamino-2-fluoro-2-acrylketone-1-yl)-4-fluoro-1-isopropyl-2-methyl-1H-benzimidazole

-
![N-[5-(4-ethylpiperazine-1-yl-methyl)pyridine-2-yl]guanidine nitrate](/upload/2024/1/4bb0ed5c-d10c-448c-b9ad-6a095b5857b4.png)
-
N-[5-(4-ethylpiperazine-1-yl-methyl)pyridine-2-yl]guanidine nitrate

-
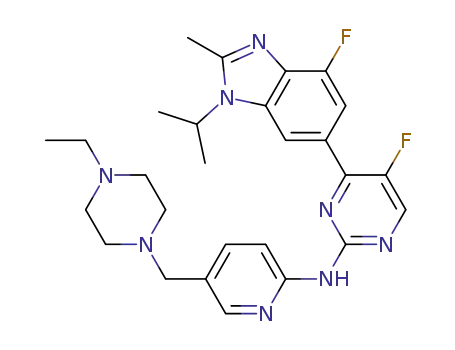
- 1231929-97-7
N-(5-((4-ethyl-1-piperazinyl)methyl)-2-pyridinyl)-5-fluoro-4-(4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl)-2-pyrimidinamine
| Conditions | Yield |
|---|---|
|
N-[5-(4-ethylpiperazine-1-yl-methyl)pyridine-2-yl]guanidine nitrate; With sulfuric acid; sodium methylate; In methanol; N,N-dimethyl-formamide; at 30 - 35 ℃; for 2h;
6-(3-N,N-dimethylamino-2-fluoro-2-acrylketone-1-yl)-4-fluoro-1-isopropyl-2-methyl-1H-benzimidazole; at 80 - 85 ℃; for 7h;
|
90.8% |
|
With sodium hydroxide; In butan-1-ol; at 90 - 100 ℃; Inert atmosphere;
|
73.1% |
-
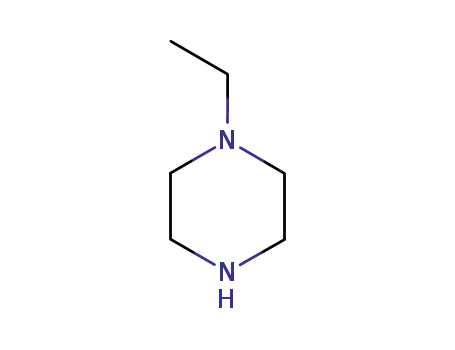
- 5308-25-8
4-ethylpiperazine

-
![6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-yl)amino)nicotinaldehyde](/upload/2024/1/a142c691-aa44-49bc-8526-28af530926de.png)
-
6-((5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-yl)amino)nicotinaldehyde

-

- 1231929-97-7
N-(5-((4-ethyl-1-piperazinyl)methyl)-2-pyridinyl)-5-fluoro-4-(4-fluoro-2-methyl-1-(1-methylethyl)-1H-benzimidazol-6-yl)-2-pyrimidinamine
| Conditions | Yield |
|---|---|
|
With formic acid; trimethyl orthoformate; In acetonitrile; at 80 ℃; for 16h; Reagent/catalyst; Sealed tube;
|
74% |
1231929-97-7 Upstream products
-
1180132-17-5
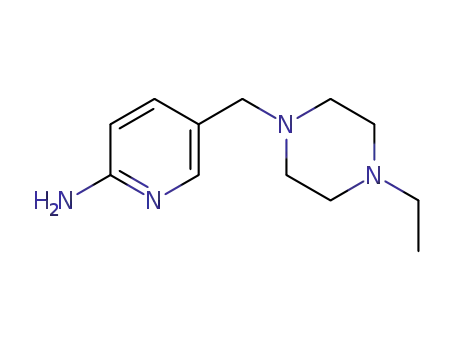
5-((4-ethylpiperazin-1-yl)methyl)-6-methylpyridin-2-amine
-
1231930-42-9
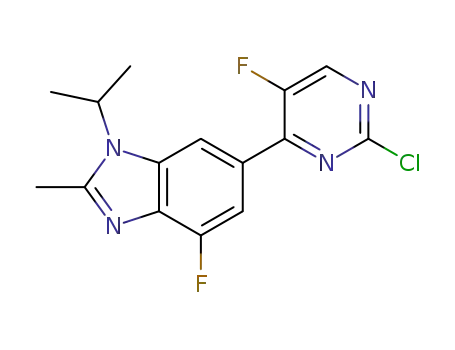
6-(2-chloro-5-fluoropyrimidin-4-yl)-4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazole
-
1231930-37-2
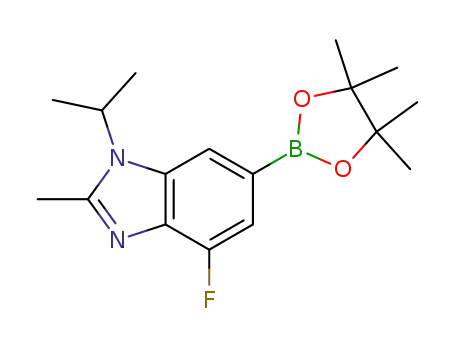
4-fluoro-1-isopropyl-2-methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-1H-benzo[d]imidazole
-
5308-25-8

4-ethylpiperazine
1231929-97-7 Downstream products
-
1231930-82-7
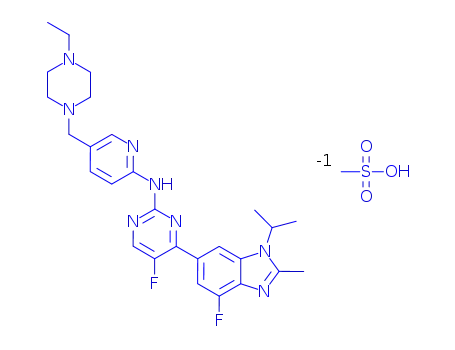
abemaciclib
-
1231930-82-7
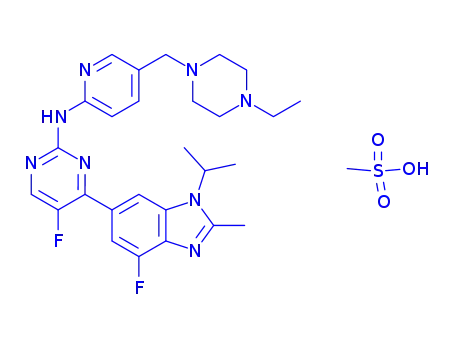
LY2835219
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Fucoidan
CAS:9072-19-9
-
Rucaparib
CAS:283173-50-2