1639208-54-0
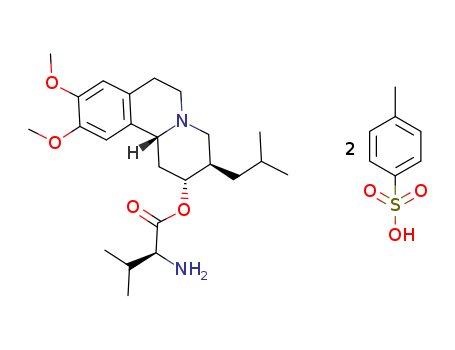
- Product Name:Valbenazine ditosylate
- Molecular Formula:C38H54N2O10S2
- Purity:99%
- Molecular Weight:762.986
Product Details:
CasNo: 1639208-54-0
Molecular Formula: C38H54N2O10S2
Chinese Factory Supply Valbenazine ditosylate,Top Purity 1639208-54-0 Customized Supply
- Molecular Formula:2C7H8O3S*C24H38N2O4
- Molecular Weight:762.986
Valbenazine ditosylate(Cas 1639208-54-0) Usage
|
Description |
Valbenazine ditosylate, also known as Valbenazine tosylate, is a medication used to treat movement disorders. It is the product ingredient for Ingrezza, marketed under the brand name Ingrezza. This medication is a vesicular monoamine transporter 2 (VMAT2) inhibitor, with a Ki (inhibition constant) ranging from 110 to 190 nM. Valbenazine is a modified metabolite of tetrabenazine, designed to be highly selective for VMAT2. |
|
Uses |
Valbenazine tosylate, the tosylate salt of valbenazine, is a vesicular monoamine transporter 2 (VMAT2) inhibitor with a Ki value of 150 nM while displaying no significant binding to VAMT1(Ki<10 μM). Ingrezza is specifically approved for the treatment of tardive dyskinesia and chorea associated with Huntington's disease. Tardive dyskinesia is a movement disorder characterized by involuntary, repetitive movements, often resulting from prolonged use of certain medications. Ingrezza offers a treatment option without causing addiction or dependence, and discontinuation typically does not lead to significant side effects. However, patients are advised to consult with their doctors before discontinuing the medication. Valbenazine, available in 40-mg and 80-mg capsules as Ingrezza, is formulated as a tosylated salt and is slightly soluble in water. Each capsule contains 73 mg or 146 mg of valbenazine tosylate, equivalent to 40 mg or 80 mg of valbenazine, respectively. The chemical structure of valbenazine is a benzoquinolizidine derivative, with a molecular formula of C38H54N2O10S2 and a molecular weight of 762.97 g/mol. The capsules should be stored at temperatures between 20° and 25° C. Valbenazine represents a valuable therapeutic option for individuals with movement disorders, providing a selective approach to VMAT2 inhibition. |
1639208-54-0 Relevant articles
PROCESSES FOR THE SYNTHESIS OF VALBENAZINE
-
Page/Page column 67-68, (2021/03/19)
The present application relates to proce...
A CRYSTALLINE FORM OF VALBENAZINE DITOSYLATE, PROCESSES FOR PREPARATION THEREOF AND USE THEREOF
-
Paragraph 0113-0115, (2021/03/19)
A crystalline form of valbenazine ditosy...
9-Cyclopropylmethoxy-dihydrotetrabenazine and its stereoisomers as vesicular monoamine transporter-2 inhibitors
Wenyan Wang , Shilan Lin, Guangying Du, Xinfa Bai, Jing Lu, Liang Ye, Hongbo Wang, Rui Zhang & Jingwei Tian
FUTURE MEDICINAL CHEMISTRYVOL. 14, NO. 13
In 2017, deutetrabenazine (a deuterated methoxy form of TBZ) and valbenazine ditosylate (VBZ) became available for the treatment of TD [12,13]. Both TBZ and deutetrabenazine are …
1639208-54-0 Process route
-
![(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-((tert-butoxycarbonyl)amino)-3-methylbutanoate](/upload/2024/1/fba8943e-ab6a-4ee5-886f-ac3f00b91d1f.png)
-
(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-((tert-butoxycarbonyl)amino)-3-methylbutanoate

-
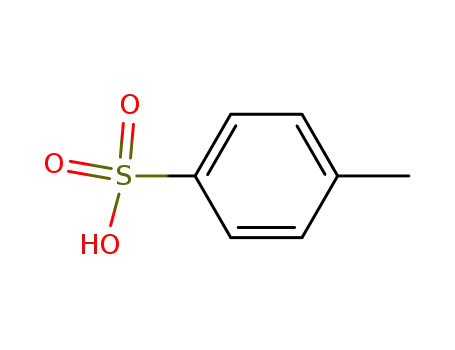
- 104-15-4
toluene-4-sulfonic acid

-
![(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl (S)-2-amino-3-methylbutanoate di(4-methylbenzenesulfonate)](/upload/2024/1/2b4b173f-eea7-492c-a22a-365f98b34d49.png)
- 1639208-54-0
(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl (S)-2-amino-3-methylbutanoate di(4-methylbenzenesulfonate)
| Conditions | Yield |
|---|---|
|
In water; at 20 ℃; for 17.5h;
|
78% |
|
(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-((tert-butoxycarbonyl)amino)-3-methylbutanoate; With hydrogenchloride; In 2-methyltetrahydrofuran; ethyl acetate; isopropyl alcohol; at 45 ℃; for 0.5h;
With hydrogenchloride; In 2-methyltetrahydrofuran; ethyl acetate; isopropyl alcohol; at 70 ℃; for 2h;
toluene-4-sulfonic acid; In acetonitrile; at 45 - 55 ℃; for 18h;
|
53% |
|
In acetonitrile; at 50 - 55 ℃; for 6h;
|
5.6 g |
|
In acetonitrile; at 20 - 65 ℃; for 14h; Large scale;
|
26.2 kg |
-
![(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate dihydrochloride](/upload/2024/1/651a234a-a5bb-4d7f-b67d-2b30f522ea79.png)
-
(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate dihydrochloride

-

- 104-15-4
toluene-4-sulfonic acid

-
![(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl (S)-2-amino-3-methylbutanoate di(4-methylbenzenesulfonate)](/upload/2024/1/2b4b173f-eea7-492c-a22a-365f98b34d49.png)
- 1639208-54-0
(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl (S)-2-amino-3-methylbutanoate di(4-methylbenzenesulfonate)
| Conditions | Yield |
|---|---|
|
(S)-(2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl 2-amino-3-methylbutanoate dihydrochloride; With sodium hydrogencarbonate; In dichloromethane; water; at 25 ℃; Large scale;
toluene-4-sulfonic acid; In acetonitrile; at 50 ℃; Solvent; Temperature; Large scale;
|
92.8% |
1639208-54-0 Upstream products
-
1025504-45-3
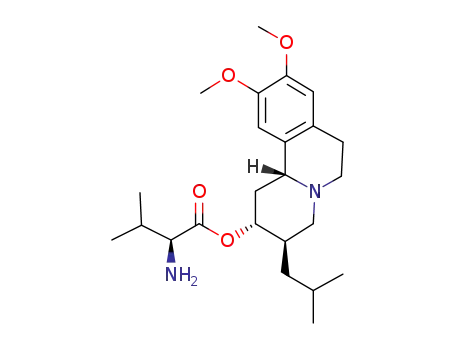
(S)-2-amino-3-methylbutyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolizin-2-yl ester
-
104-15-4

toluene-4-sulfonic acid
-
91342-74-4
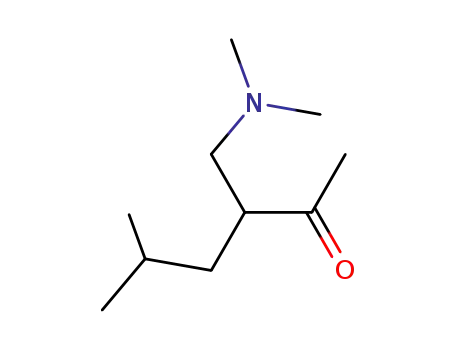
3-[(dimethylamino)methyl]-5-methylhexan-2-one
-
3466-75-9
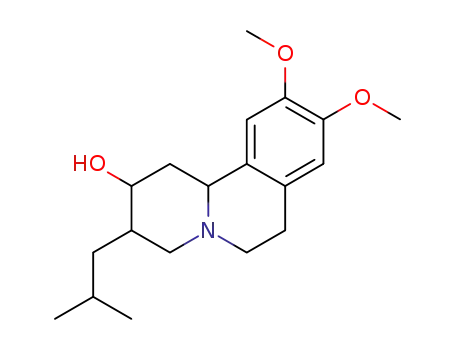
Dihydrotetrabenazine
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
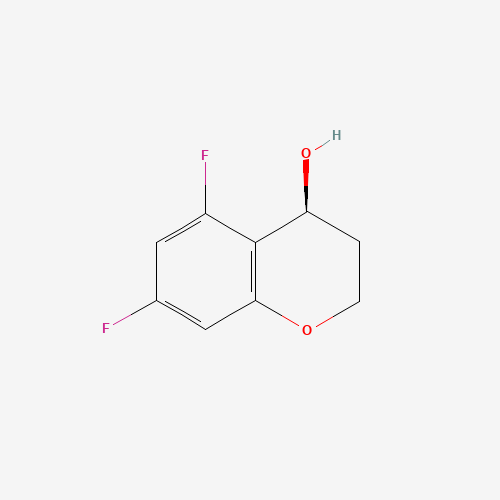
(S)-5,7-difluorochroman-4-ol
CAS:942195-91-7
-
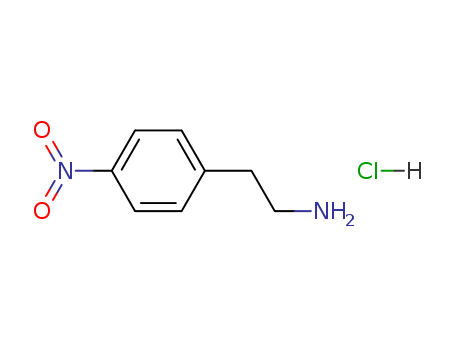
4-Nitrophenethylamine hydrochloride
CAS:29968-78-3