942195-55-3
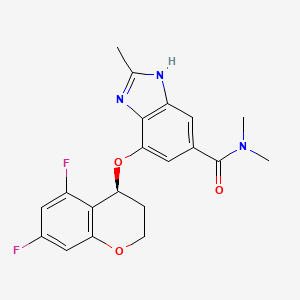
- Product Name:Tegoprazan
- Molecular Formula:C20H19F2N3O3
- Purity:99%
- Molecular Weight:0
Product Details:
CasNo: 942195-55-3
Molecular Formula: C20H19F2N3O3
Buy High Grade Tegoprazan,High Purity 942195-55-3 Best Price
- Molecular Formula:
- Molecular Weight:0
Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers.
Tegoprazan(Cas 942195-55-3) Usage
|
Description |
Tegoprazan is a novel potassium-competitive acid blocker (P-CAB) designed for the treatment of acid-related gastrointestinal diseases such as gastroesophageal reflux disease (GERD), peptic ulcers, erosive esophagitis (EE), and nonerosive reflux disease (NERD). Unlike traditional proton pump inhibitors (PPIs), Tegoprazan blocks the potassium-binding site of gastric H+/K+ ATPase, offering a more potent and reversible suppression of gastric acid. |
|
Uses |
Approved in South Korea in 2018 under the brand name K-CAB®, Tegoprazan has since expanded to markets in China, the Philippines, and Mongolia. It has demonstrated strong efficacy in preclinical studies, showing rapid absorption and higher concentration in gastric tissues compared to plasma. Tegoprazan inhibits gastric acid secretion induced by histamine and pentagastrin in animal models, offering potential benefits for patients with motility-impaired conditions. Its innovative mechanism provides a promising alternative for the treatment of gastric acid-related disorders. |
| Referances |
Tegoprazan, a Novel Potassium-Competitive Acid Blocker to Control Gastric Acid Secretion and Motility , Nobuyuki Takahashi and Yukinori Take Tegoprazan potently inhibited histamine-induced gastric acid secretion in dogs, and a complete inhibition was observed at 1.0 mg/kg starting from 1 hour after administration. Moreover, an oral administration of tegoprazan at 1 and 3 mg/kg reversed the pentagastrin-induced acidified gastric pH to the neutral range. Interestingly, 3 mg/kg tegoprazan immediately evoked a gastric phase III contraction of the migrating motor complex in pentagastrin-treated dogs and similar effects was observed with the other P-CAB, vonoprazan. Tegoprazan is the novel P-CAB that may provide a new option for the therapy of gastric acid–related and motility-impaired diseases. |
942195-55-3 Relevant articles
Comparison of pharmacokinetic characteristics of two Tegoprazan (CJ-12420) formulations in healthy male subjects
JG Hwang, H Yoo, JW Lee, GS Song
TCP Transl Clin Pharmacol, 2019;27(2):80-85
As a new treatment option, potassium-competitive acid blockers (P-CABs), such as tegoprazan, have been developed. This study was performed to compare the pharmacokinetics (PKs) between two formulations (test and reference drugs) of tegoprazan 100 mg tablets …
Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects
Sungpil Han, Hee Youn Choi, Yo Han Kim, Ji Yeon Nam, Bongtae Kim, Geun Seog Song, Hyeong-Seok Lim, Kyun-Seop Bae
Alimentary Pharmacology & Therapeutics, Volume50, Issue7 October 2019 Pages 751-759
A phase I, randomised, double-blind and placebo-controlled clinical trial was conducted in 56 healthy male subjects without Helicobacter pylori infection. In the single ascending dose study, 50, 100, 200 and 400 mg tegoprazan were administered to 32 subjects. In the multiple ascending dose study, 100 and 200 mg tegoprazan were administered every 24 hours to each of the eight subjects for 7 days.
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Avatrombopag maleate
CAS:677007-74-8
-
Ixazomib citrate
CAS:1201902-80-8