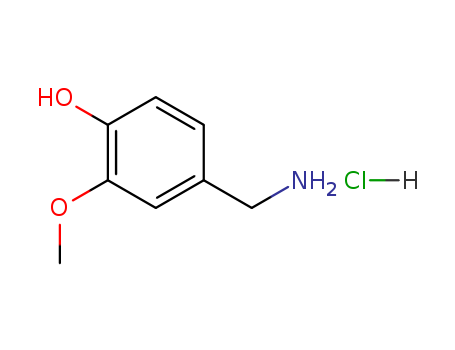
7149-10-2
- Product Name:Vanillylamine Hcl
- Molecular Formula:C8H12ClNO2
- Purity:99%
- Molecular Weight:189.642
Product Details:
CasNo: 7149-10-2
Molecular Formula: C8H12ClNO2
Appearance: white to light yellow crystal powder
Chinese Factory Supply Best Quality Vanillylamine Hcl 7149-10-2 Best Price
- Molecular Formula:C8H12ClNO2
- Molecular Weight:189.642
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00102mmHg at 25°C
- Melting Point:219-221 °C (dec.)(lit.)
- Boiling Point:292.9 °C at 760 mmHg
- Flash Point:130.9 °C
- PSA:55.48000
- LogP:2.36180
Vanillylamine Hcl(Cas 7149-10-2) Usage
|
Description |
Vanillylamine Hydrochloride, also known as Vanillylamine HCl, is a chemical compound obtained as a metabolite of Capsaicin. It serves as a biochemical reagent and organic compound utilized in various research applications within the life sciences field. |
|
Uses |
Vanillylamine Hydrochloride serves as a valuable reagent in biochemical research, particularly in studies related to metabolism, cellular signaling, and pharmacology. Its inclusion in synthetic pathways enables the creation of novel substances with potential applications in pharmaceuticals, agrochemicals, and materials science. |
| Preparation Method |
Initial Reaction: 3-methoxy-4-hydroxybenzaldehyde oxime is added to a reaction container, along with alcohol and a composite catalyst. Hydrogen gas is introduced under normal pressure conditions to protect the reaction, followed by heating to facilitate the reaction process. Filtration: After completion of the reaction, the mixture is filtered to remove any solid impurities or by-products, yielding a filtrate containing the desired compound. Acidification: Hydrochloric acid with a mass percentage concentration of 30% is added to the filtrate to adjust its pH value to approximately 1. This step facilitates the precipitation of Vanillylamine Hydrochloride as a white crystal. Drying: The precipitated Vanillylamine Hydrochloride crystals are filtered out and subjected to vacuum drying to remove any remaining moisture and solvents, resulting in the final product. |
InChI:InChI=1/C8H11NO2/c1-11-8-4-6(5-9)2-3-7(8)10/h2-4,10H,5,9H2,1H3/p+1
7149-10-2 Relevant articles
Fabrication of ω-Transaminase@Metal-Organic Framework Biocomposites for Efficiently Synthesizing Benzylamines and Pyridylmethylamines
Chen, Lina,Ding, Yingying,Jiao, Qingcai,Liu, Junzhong,Yu, Jinhai,Zhang, Hongjuan,Zong, Weilu
, (2021/11/05)
In this study, ten ω-transaminases (ω-TA...
Elongation of the Hydrophobic Chain as a Molecular Switch: Discovery of Capsaicin Derivatives and Endogenous Lipids as Potent Transient Receptor Potential Vanilloid Channel 2 Antagonists
Schiano Moriello, Aniello,López Chinarro, Silvia,Novo Fernández, Olalla,Eras, Jordi,Amodeo, Pietro,Canela-Garayoa, Ramon,Vitale, Rosa Maria,Di Marzo, Vincenzo,De Petrocellis, Luciano
, p. 8255 - 8281 (2018/09/25)
The transient receptor potential vanillo...
Synthesis and elucidation of deuterated vanillylamine hydrochloride and capsaicin
Sang Wook Kim, Jeong Hoon Park, Soon Jae Jung, Tae Bum Choi, Min Goo Hur, Seung Dae Yang, Kook Hyun Yu
Journal of Labelled Compounds and Radiopharmaceuticals, Volume52, Issue13 November 2009 Pages 563-565
Although the biosynthesis of capsaicin is known to involve the condensation of vanillylamine and 8-methylnonenoic acid by capsaicin synthase, the mechanism of biosynthesis is still not fully understood. In this study, deuterium labelled versions of capsaicin and the precursor vanillylamine were synthesized in order to investigate the biosynthesis of capsaicin in hot peppers.
7149-10-2 Upstream products
-
2874-33-1
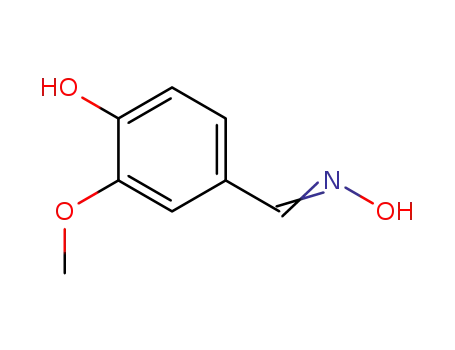
vanillin oxime
-
93249-67-3
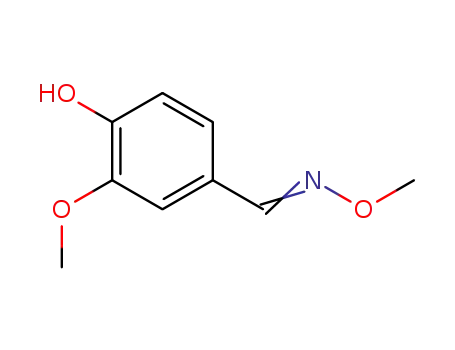
4-hydroxy-3-methoxybenzaldehyde O-methyloxime
-
121-33-5
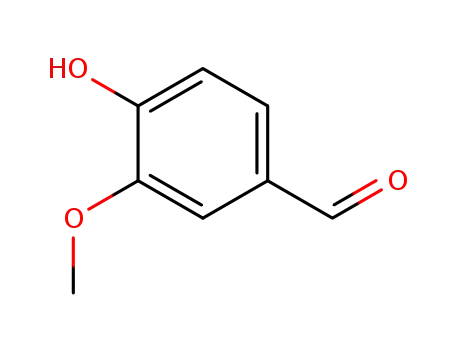
vanillin
-
134283-49-1
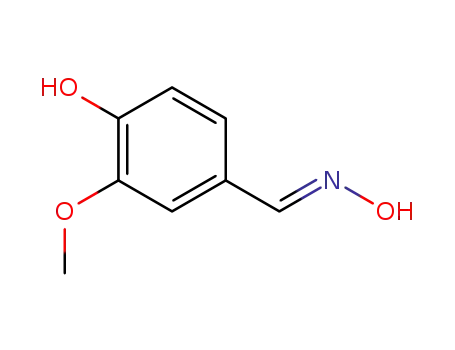
(E)-4-hydroxy-3-methoxybenzaldehyde oxime
7149-10-2 Downstream products
-
89575-10-0
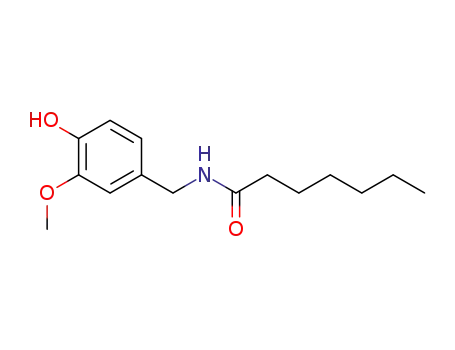
N-heptanoate vanillylamide
-
58493-49-5
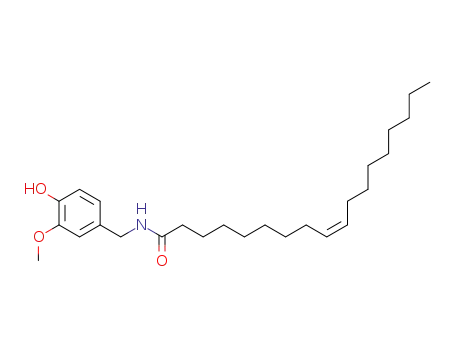
olvanil
-
58493-47-3
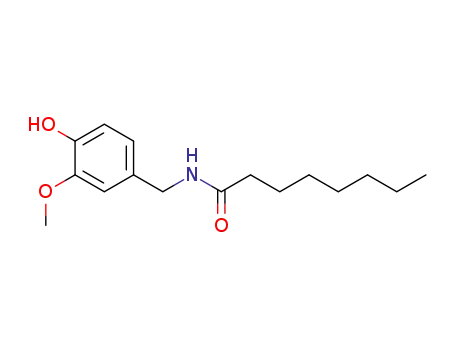
Caprylic acid vanillylamide
-
31078-36-1
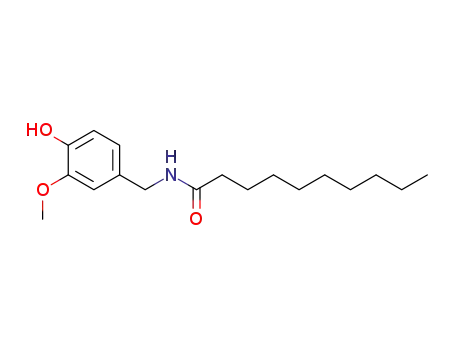
Vanillyl N-decoylamide
Relevant Products
-
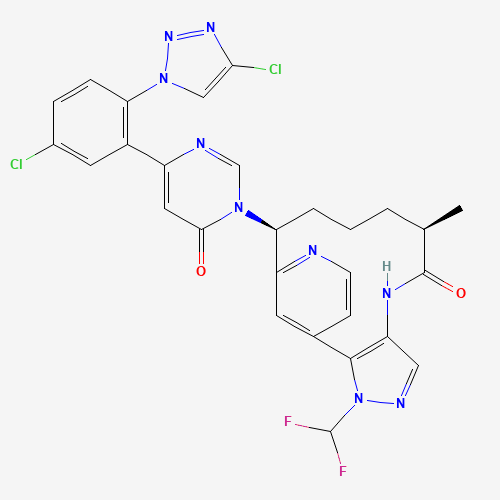
Milvexian
CAS:1802425-99-5
-
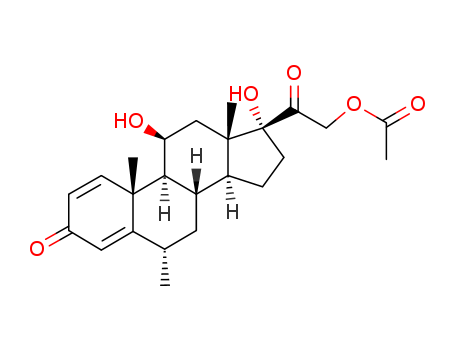
Methyl Prednisolone Acetate
CAS:53-36-1
-
Gemfibrozil
CAS:25812-30-0