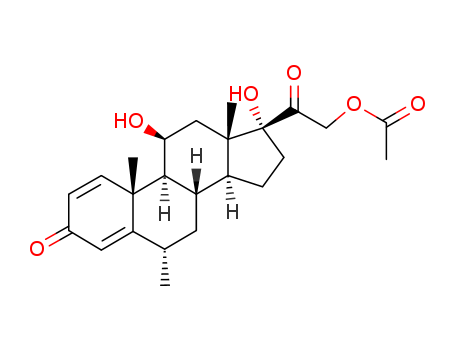
53-36-1
- Product Name:Methyl Prednisolone Acetate
- Molecular Formula:C24H32O6
- Purity:99%
- Molecular Weight:416.514
Product Details:
CasNo: 53-36-1
Molecular Formula: C24H32O6
Appearance: Off-White Solid
Chinese Factory Supply Top Purity Methyl Prednisolone Acetate 53-36-1 Cheap Price
- Molecular Formula:C24H32O6
- Molecular Weight:416.514
- Appearance/Colour:Off-White Solid
- Vapor Pressure:5.41E-16mmHg at 25°C
- Melting Point:206oC
- Refractive Index:1.58
- Boiling Point:582.5 °C at 760 mmHg
- PKA:12.41±0.70(Predicted)
- Flash Point:196.5 °C
- PSA:100.90000
- Density:1.26 g/cm3
- LogP:2.37440
Methylprednisolone acetate(Cas 53-36-1) Usage
|
Description |
Methylprednisolone acetate (MPA) is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, specifically the C21 acetate ester of methylprednisolone. It is widely used in clinical and veterinary medicine for its anti-inflammatory and immunosuppressive properties. Methylprednisolone acetate is sold under various brand names, including Depo-Medrol. |
|
Uses |
Methylprednisolone acetate is primarily used to treat pain and inflammation associated with various musculoskeletal disorders, including arthritis. It is also used for the symptomatic relief of dysmenorrhea, a condition characterized by painful menstrual periods. Additionally, it may be used for other conditions as determined by a healthcare provider. |
|
Brand name |
Methylprednisolone acetate is available under various brand names, with Depo-Medrol being one of the most commonly recognized brands. |
InChI:InChI=1/C24H32O6/c1-13-9-16-17-6-8-24(29,20(28)12-30-14(2)25)23(17,4)11-19(27)21(16)22(3)7-5-15(26)10-18(13)22/h5,7,10,13,16-17,19,21,27,29H,6,8-9,11-12H2,1-4H3
53-36-1 Relevant articles
Formulation and Evaluation of Pharmaceutically Equivalent Parenteral Depot Suspension of Methyl Prednisolone Acetate
A. Alam, MD., Alka Ahuja, Sanjula Baboota, S. K. Gidwani,1 and J. Ali*
, Indian J Pharm Sci. 2009 Jan-Feb; 71(1): 30–34.
For the analysis of methyl prednisolone acetate by HPTLC, pre- coated silica gel 60 F (254) plates (E. Merck India, Ltd.) were selected Formic acid, ethyl acetate, toluene, methanol, acetonitrile, diethyl ether were used separately as neat solvents. Developed chromatograms were then visualized in iodine chamber for the detection of methyl prednisolone acetate spots.
Dangers From Methylprednisolone Acetate Therapy by Intraspinal Injection
Dewey A. Nelson, MD
, Archives of neurology, 1988
Clinical trials first began in 1960 with methylprednisolone acetate (Depo-Medrol) administered intrathecally, in an attempt to treat both disk disease and multiple sclerosis. After a few reports of salubrious results, there began an out-pouring of contradictory data, which continues in 1988.
Intra-articular steroids in knee osteoarthritis: a comparative study of triamcinolone hexacetonide and methylprednisolone acetate
Debasish Pyne, Yiannakis Ioannou, Ramesh Mootoo & Asgar Bhanji
, Clinical Rheumatology, Volume 23, pages 116–120, (2004)
The aim of this study was to compare the effectiveness of triamcinolone hexacetonide (THA) and methylprednisolone acetate (MPA), given via the intra-articular route at equipotent dosage to patients with symptomatic knee OA with effusion, in a double-blind randomized comparative trial.
53-36-1 Downstream products
-
83-43-2
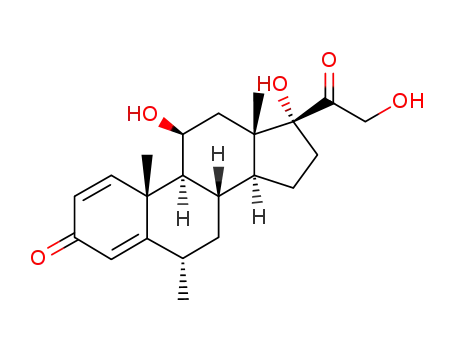
Methylprednisolone
-
86401-94-7
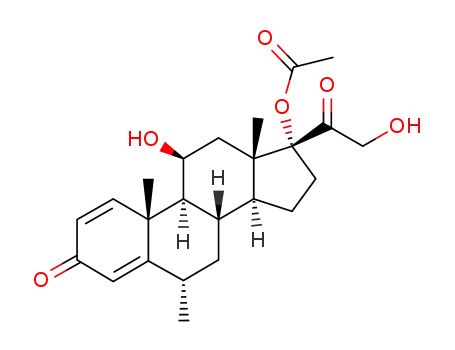
17α-acetoxy-11β,21-dihydroxy-6α-methyl-1,4-pregnadiene-3,20-dione
-
6870-94-6
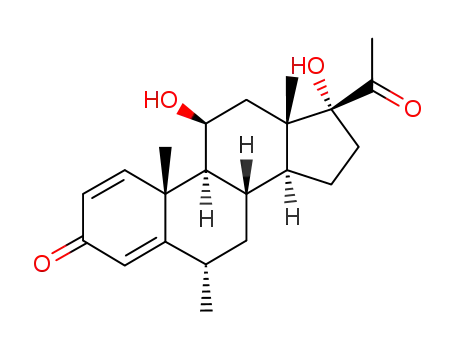
11β,17α-dihydroxy-6α-methyl-1,4-pregnadiene-3,20-dione
-
61919-52-6
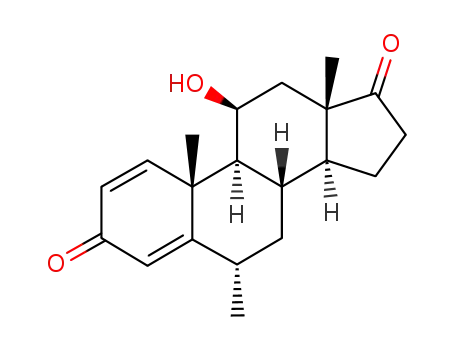
11β-hydroxy-6α-methyl-1,4-androstadiene-3,17-dione
Relevant Products
-
Decanoyl/octanoyl-glycerides
CAS:65381-09-1
-
Carbidopa
CAS:38821-49-7
-
Vanillylamine Hcl
CAS:7149-10-2