38821-49-7
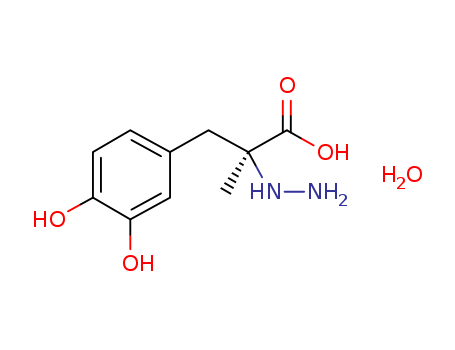
- Product Name:Carbidopa
- Molecular Formula:C10H16N2O5
- Purity:99%
- Molecular Weight:244.247
Product Details:
CasNo: 38821-49-7
Molecular Formula: C10H16N2O5
Buy Reliable Quality Carbidopa,Sale 38821-49-7 Safe Delivery
- Molecular Formula:C10H14N2O4*H2O
- Molecular Weight:244.247
- Vapor Pressure:5.27E-12mmHg at 25°C
- Boiling Point:528.7 °C at 760 mmHg
- Flash Point:273.5 °C
- PSA:125.04000
- Density:1.42 g/cm3
- LogP:0.97380
Carbidopa(Cas 38821-49-7) Usage
|
Chemical Properties |
white or yellowish-white powder. almost odorless. combined with levodopa for Parkinson's disease and Parkinson's syndrome. |
|
Description |
Carbidopa is a medication that prevents the breakdown of levodopa before it reaches the brain. It belongs to the class of aromatic L-amino acid decarboxylase inhibitors. By inhibiting the enzyme that converts levodopa into dopamine outside the brain, carbidopa allows more levodopa to reach the brain, where it is converted into dopamine, alleviating Parkinson’s disease symptoms. |
|
Uses |
Carbidopa is classified as a decarboxylase inhibitor and is used in the treatment of Parkinson’s disease. It is commonly used in combination with levodopa to enhance the treatment's effectiveness. Carbidopa is primarily used in combination with levodopa to treat Parkinson’s disease and parkinsonism. The combination therapy reduces symptoms such as tremors, muscle stiffness, and impaired movement control. By preventing the breakdown of levodopa outside the brain, carbidopa increases the effectiveness of levodopa at lower doses. This reduces side effects like nausea and vomiting, which are often associated with high doses of levodopa. |
|
Brand name |
Lodosyn (Bristol-Myers Squibb): Available in combination with levodopa under the brand name Sinemet. |
| Synthesis | Carbidopa is synthesized through a multi-step chemical process that involves the formation of aromatic L-amino acids. The process incorporates protecting groups and is designed to produce an enantiomerically pure product, which is important for its therapeutic effectiveness. |
InChI:InChI=1/C10H14N2O4.H2O/c1-10(12-11,9(15)16)5-6-2-3-7(13)8(14)4-6;/h2-4,12-14H,5,11H2,1H3,(H,15,16);1H2
38821-49-7 Relevant articles
Comparison of Immediate-Release and Controlled Release Carbidopa/Levodopa in Parkinson’s Disease: A Multicenter 5-Year Study
Gilbert Block; Charles Liss; Scott Reines; Joseph Irr; Donald Nibbelink
Eur Neurol (1997) 37 (1): 23–27.
During a 5-year treatment period, control of parkinsonian symptoms was maintained by both immediate-release and sustained-release carbidopa/levodopa. Both treatment regimens were associated with a low incidence of motor fluctuations and dyskinesias. There was a statistically significant difference (p < 0.05) in activities of daily living as measured by the UPDRS in favor of Sinemet CR....
Levodopa with benserazide or carbidopa in Parkinson disease
U. K. Rinne, M.D., and P. Mölsä, M.D.
, Neurology,December 1979 issue 29 (12) 1584-1589
In pretrial studies of plasma levodopa responses, 200 mg of levodopa and 50 mg of benserazide was equal to 250 mg of levodopa combined with 25 mg of carbidopa. Equal plasma levodopa responses to both combinations were also found during the trial.
The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: The mechanism of action in the treatment of parkinsonism
Dr. John G. Nutt MD, William R. Woodward PhD, John L. Anderson MS
, Annals of neurology, Volume18, Issue5 November 1985 Pages 537-543
Carbidopa reduced by 50% both the infusion rate required to produce a clinical response and the time required for plasma clearance of levodopa. Using this value for clearance, it is estimated that carbidopa doubles the bioavailability of orally administered levodopa.
38821-49-7 Upstream products
-
37178-37-3
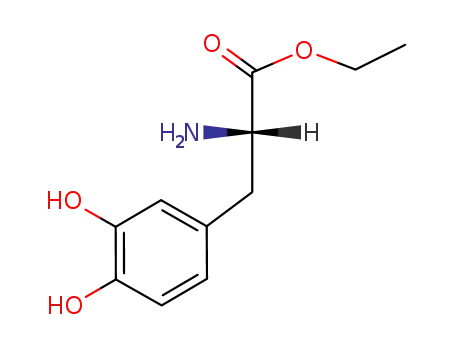
L-DOPA ethyl ester
-
1426847-87-1
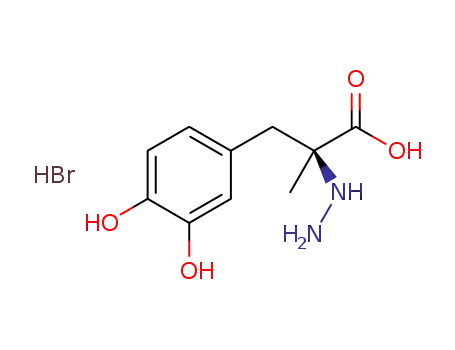
(S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid hydrobromide
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Ondansetron Hcl
CAS:103639-04-9
-
Methyl Prednisolone Acetate
CAS:53-36-1