577778-58-6
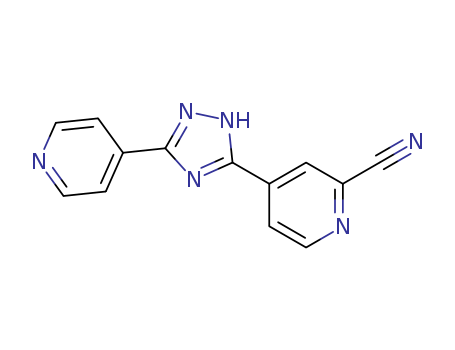
- Product Name:Topiroxostat
- Molecular Formula:C13H8N6
- Purity:99%
- Molecular Weight:248.247
Product Details:
CasNo: 577778-58-6
Molecular Formula: C13H8N6
Buy High Grade Topiroxostat,Factory Sells 577778-58-6 Efficient Shipping
- Molecular Formula:C13H8N6
- Molecular Weight:248.247
- Boiling Point:594.7±60.0 °C(Predicted)
- PKA:7.47±0.10(Predicted)
- PSA:91.14000
- Density:1.45±0.1 g/cm3(Predicted)
- LogP:1.80038
Topiroxostat (Cas 577778-58-6) Usage
|
Description |
Topiroxostat is a selective xanthine oxidase inhibitor medication used in the treatment and management of hyperuricemia and gout. It works by significantly lowering serum urate levels, thus reducing the risk of gout attacks and preserving renal function in certain patients. |
| Classification and Trade Names | Topiroxostat is classified as a xanthine oxidase inhibitor. It is available under the trade names Topiloric and Uriadec. |
| Indications | It is indicated for the treatment of gout and hyperuricemia. |
| Clinical Use | Topiroxostat is primarily indicated for the treatment of gout, a form of arthritis caused by elevated levels of uric acid in the blood. By inhibiting the enzyme involved in uric acid synthesis, it helps reduce serum uric acid concentrations, alleviating symptoms and preventing gout attacks. |
| Approval and Availability | Topiroxostat was approved for use in Japan in June 2013. It is considered a safe and effective medication for the treatment of gout and hyperuricemia in daily practice. |
577778-58-6 Relevant articles
Apoptosis induced by an uromodulin mutant C112Y and its suppression by topiroxostat
Sulistiyati Bayu Utami, Endang Mahati, Peili Li
, Clinical and Experimental Nephrology, Volume 19, pages 576–584, (2015)
The half-life of C112Y was shortened and it was restored by a proteasome inhibitor MG132. Immunofluorescence revealed decreased levels of C112Y in the Golgi apparatus and on the plasma membrane. Expression of C112Y induced cellular apoptosis as revealed by flow cytometry. Apoptosis induced by C112Y was suppressed by topiroxostat.
Clinical efficacy and safety of topiroxostat in Japanese hyperuricemic patients with or without gout: a randomized, double-blinded, controlled phase 2b study
Tatsuo Hosoya, Tomomitsu Sasaki & Tetsuo Ohashi
Clinical Rheumatology, Volume 36, pages 649–656, (2017)
During the 16-week study, 157 Japanese hyperuricemic patients with or without gout were randomly assigned to receive a placebo, topiroxostat at 120 or 160 mg/day, or allopurinol at 200 mg/day. The primary endpoint of this study was to determine the lowering rate of serum uric acid levels compared to those of baseline at the end of administration.
577778-58-6 Upstream products
-
100-48-1
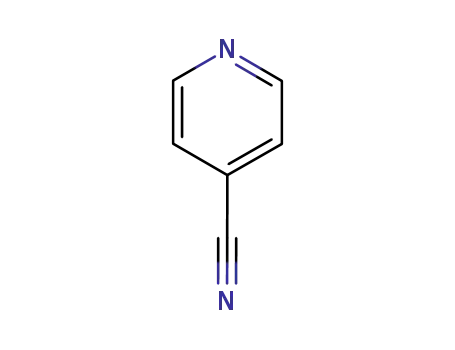
pyridine-4-carbonitrile
-
94413-64-6
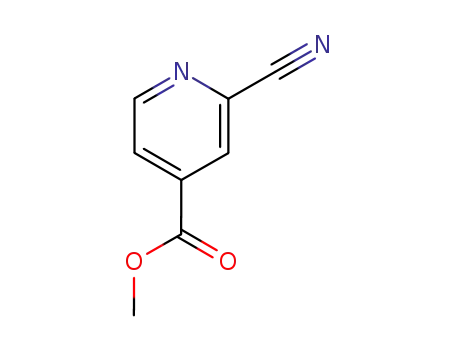
2-Cyano-4-carbomethoxypyridine
577778-58-6 Downstream products
-
577778-88-2
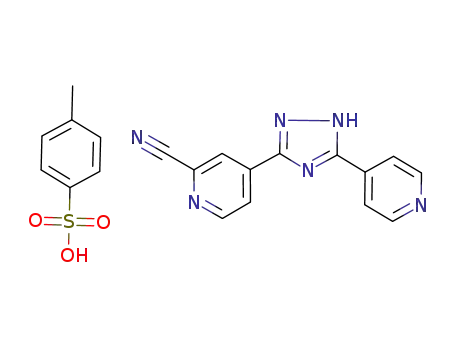
4-[5-(pyridin-4-yl)-1H-1,2,4-triazol-3-yl]pyridine-2-carbonitrile p-toluenesulfonate
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Azilsartan Medoxomil Potassium
CAS:863031-24-7