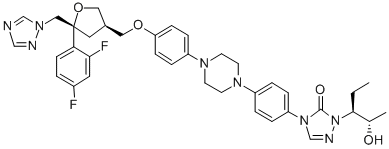
171228-49-2
- Product Name:Posaconazole
- Molecular Formula:C37H42F2N8O4
- Purity:99%
- Molecular Weight:700.78
Product Details:
CasNo: 171228-49-2
Molecular Formula: C37H42F2N8O4
Purity: 99%
Pharmacological effects
Posaconazole (posaconazole) is derived from itraconazole. It is currently subject to III phase clinical trials. Its pharmacological effects are similar with azoles, but compared with itraconazole, it has a stronger inhibitory effect on the C14 demethylation of steroid, especially for Aspergillus.
Pharmacokinetics
Studies on dosage and dosage protocol have shown that the rate of absorption and elimination rate is in line with the single-compartment model. There are significant differences on the relative bioavailability of different doses of oral suspension. It can be taken separately (every 12 hours or every 6 hours) which can significantly improve the bioavailability with the protein binding rate of 98% to 99%. With respect to tablets, the bioavailability of suspensions increase and food can significantly improve the speed and extent of absorption of drug absorption. An investigation of renal dysfunction on the pharmacokinetics of the drug study results has showed that the drug can’t be removed by hemodialysis without being affected by hemodialysis. Single-dose study has showed that patients with varying degrees of chronic kidney disease have no necessity for dosage adjustment. The Half-life of this is about 25 hours which can be primarily metabolized by the liver.
Clinical indications and usage
It can be clinically used for the treatment of aspergillosis, zygnmycosis, and fusariumsis and can also be used for infection caused by part of fluconazole-resistant Candida genus. Studies have shown that posaconazole can widely and effectively applied to the treatment of phaeohyphomycosis and improve the infection survival rate of dermatitidis infection in a dose-dependent manner. The drug, as second-line drugs, has an effective rate of 44% to 78% against the invasive aspergillosis which is resistant to amphotericin B and itraconazole. It also has an effective rate of 71% against the zygomycete fungi. The drug is an oral suspension with the recommended dose of 200mg and 4 times per day with meals and taken orally for 7 to 10 days. This dose can also be maintained or changed to 400mg with oral administration of 2 times per day. The steady-state plasma concentration can reach within 7 to 10 days.
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
S-Linagliptin
CAS:668270-11-9