1375073-94-1
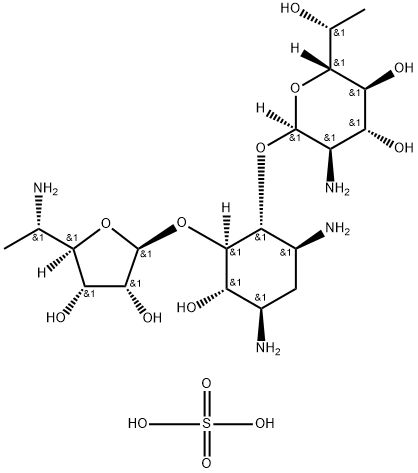
- Product Name:Exaluren sulfate
- Molecular Formula:C19H40N4O14S
- Purity:99%
- Molecular Weight:580.6
Product Details:
CasNo: 1375073-94-1
Molecular Formula: C19H40N4O14S
Appearance: Powder
Purity: 99%
Factory Supply High Purity Exaluren sulfate,Top Purity 99% 1375073-94-1 Efficient Shipping
Exaluren (ELX-02) sulfate is an investigational, advanced synthetic eukaryotic ribosome selective glycoside (ERSG). Exaluren sulfate is being developed as a therapy for genetic diseases caused by nonsense mutations[1].
Exaluren (ELX-02) sulfate (10 and 30 mg/kg; repeat subcutaneous administration; twice weekly, total of 8 doses) shows accumulation in tissues that is dose dependent without gender difference[1].
In plasma Exaluren (ELX-02) sulfate is rapidly absorbed with a Tmax of 0.25 h after both single (a single subcutaneous injection at 10 mg/kg at dose volume of 5 mL/kg) and repeated administration (twice weekly with 10 mg/kg/dose for 21 days; total of 7 administrations). Exaluren (ELX-02) sulfate is rapidly eliminated from plasma in a biphasic manner with the terminal half-life (T1/2) of 0.5 h[1].
In a CtnsY226X nonsense mutant mouse, subcutaneous Exaluren (ELX-02) sulfate accumulates in kidney tissue without overt renal toxicity and that Exaluren (ELX-02) sulfate (10 mg/kg X2/week for 3 weeks) reduces renal cystine accumulation in vivo[1].
| NCT Number | Sponsor | Condition | Start Date | Phase |
|---|---|---|---|---|
| NCT05448755 | Eloxx Pharmaceuticals, Inc. |
Alport Syndrome |
November 28, 2022 | Phase 2 |
| NCT04069260 | Eloxx Pharmaceuticals, Inc. |
Genetic Disease|Nonsense Mutation|Cystinosis |
August 2, 2019 | Phase 2 |
| NCT03309605 | Eloxx Pharmaceuticals, Inc.|SGS Life Sciences, a division of SGS Belgium NV |
Genetic Disease|Nonsense Mutation |
October 11, 2017 | Phase 1 |
| NCT04126473 | Eloxx Pharmaceuticals, Inc. |
Cystic Fibrosis |
November 5, 2019 | Phase 2 |
| NCT04135495 | Eloxx Pharmaceuticals, Inc. |
Cystic Fibrosis |
November 25, 2019 | Phase 2 |
| NCT03292302 | Eloxx Pharmaceuticals, Inc.|SGS Life Sciences, a division of SGS Belgium NV |
Genetic Disease|Nonsense Mutation |
September 26, 2017 | Phase 1 |
| NCT02807961 | Eloxx Pharmaceuticals, Inc. |
Genetic Diseases|Nonsense Mutations |
July 2016 | Phase 1 |
| NCT03776539 | Eloxx Pharmaceuticals, Inc.|Syneos Health |
Impaired Renal Function |
January 4, 2019 | Phase 1 |
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Pirtobrutinib (LOXO-305)
CAS:2101700-15-4
-
Pevonedistat Hcl
CAS:1160295-21-5