540737-29-9
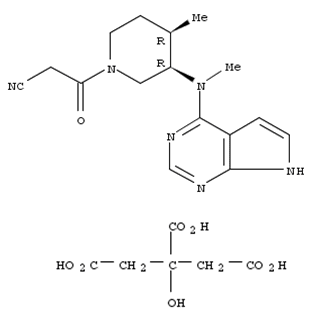
- Product Name:Tofacitinib Citrate
- Molecular Formula:C16H20N6O·C6H8O7
- Purity:99%
- Molecular Weight:504.50
Product Details:
CasNo: 540737-29-9
Molecular Formula: C16H20N6O·C6H8O7
Quality Factory Supply Tofacitinib Citrate,Sale 540737-29-9 Fast Delivery
- Molecular Formula:C16H20N6O·C6H8O7
- Molecular Weight:504.50
- Melting Point:201 °C (decomp)
- Boiling Point:585.8 °C at 760 mmHg
- Flash Point:308.1 °C
- PSA:221.04000
- LogP:0.23418
Tofacitinib citrate(Cas 540737-29-9) Usage
|
Description |
Tofacitinib Citrate is the citrate salt form of tofacitinib, a Janus kinase (JAK) inhibitor. It is primarily used to treat inflammatory conditions such as rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and ankylosing spondylitis. Tofacitinib works by inhibiting the JAK-STAT signaling pathway, which plays a crucial role in the inflammatory and immune response. |
| Administration and Formulation | Immediate-release tablets: 5 mg, taken twice a day. Extended-release tablets: 11 mg, taken once a day. Oral solution: Available for patients with difficulty swallowing tablets. |
|
Uses |
Rheumatoid Arthritis (RA): Used to treat moderate to severe rheumatoid arthritis in adults who have not responded to or are intolerant of methotrexate. It is often used as a disease-modifying antirheumatic drug (DMARD). Psoriatic Arthritis (PsA): Effective in treating adults with active psoriatic arthritis, reducing joint inflammation and symptoms associated with the condition. |
| Side Effects and Risks | Infections: Increased risk of serious infections such as tuberculosis, fungal infections, and viral infections due to suppression of the immune system. Blood Clots: Tofacitinib has been associated with an increased risk of deep vein thrombosis (DVT) and pulmonary embolism (PE), especially in patients on higher doses. Cardiovascular Issues: Some studies have suggested a possible association with increased risk of heart attack and stroke in certain patients. Cancer: Long-term use has been linked to an increased risk of certain cancers, including lymphoma and skin cancer. Weight Gain: Though uncommon, weight gain has been reported as a side effect in some studies. |
InChI:InChI=1S/C16H20N6O.C6H8O7/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16;7-3(8)1-6(13,5(11)12)2-4(9)10/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20);13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t11-,13+;/m1./s1
540737-29-9 Relevant articles
Development of a robust, environmentally responsible process for the manufacture of tofacitinib citrate
R Vaidyanathan
Scalable Green Chemistry: Case Studies from the Pharmaceutical Industry
Process development efforts that resulted in a robust, environmentally responsible, commercializable process for tofacitinib citrate will be presented in this chapter.
Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata
Milène Kennedy Crispin,1 Justin M. Ko,1 Brittany G. Craiglow,2,3 Shufeng Li,1 Gautam Shankar,1 Jennifer R. Urban,4 James C. Chen,5,6 Jane E. Cerise,5 Ali Jabbari,5 Mårten C.G. Winge,1 M. Peter Marinkovich,1 Angela M. Christiano,5,7 Anthony E. Oro,1 and Brett A. King2
, JCI Insight. 2016 Sep 22; 1(15): e89776.
This was a 2-center, open-label, single-arm trial using the pan-JAK inhibitor, tofacitinib citrate, for AA with >50% scalp hair loss, alopecia totalis (AT), and alopecia universalis (AU). Tofacitinib (5 mg) was given twice daily for 3 months. Endpoints included regrowth of scalp hair, as assessed by the severity of alopecia tool (SALT), duration of hair growth after completion of therapy, and disease transcriptome.
Tofacitinib (Xeljanz): The First-in-Class JAK Inhibitor for the Treatment of Rheumatoid Arthritis
Robert W. Dugger, Mark E. Flanagan, Rajappa Vaidyanathan
Innovative Drug Synthesis, 16 November 2015
Tofacitinib operates by inhibiting the JAK pathways utilized by several cytokines implicated in the pathogenesis of the disease. The SAR associated with the tofacitinib discovery program has been reported on extensively. Key elements of this medicinal chemistry program include the application of high-throughput-screening (HTS) to identify a lead molecule (5); and the early use of high-speed analoging (HSA).
540737-29-9 Upstream products
-
384336-73-6
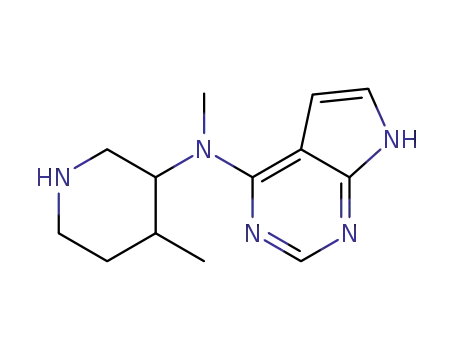
(3R,4R)-methyl-(4-methyl-piperidin-3-yl)-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine
-
56657-76-2
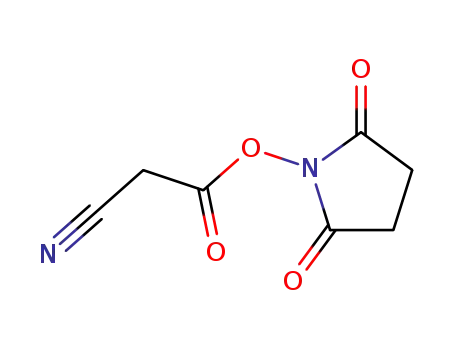
succinimidyl cyanoacetate
-
77-92-9
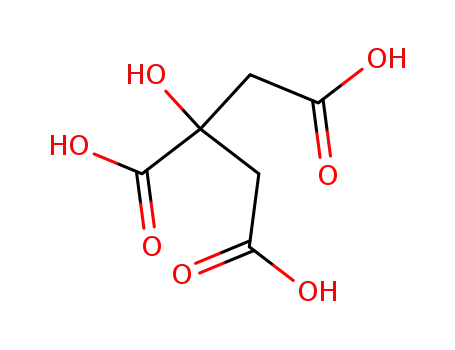
citric acid
-
477600-74-1
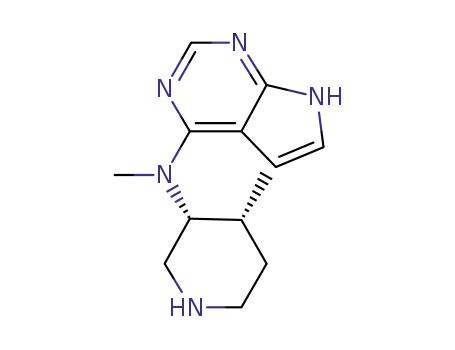
tert-butyl (3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidine-1-carboxylate
Relevant Products
-
Diatrizoic Acid
CAS:50978-11-5
-
Amikacin Sulphate
CAS:39831-55-5
-
Linagliptin
CAS:668270-12-0