203787-91-1
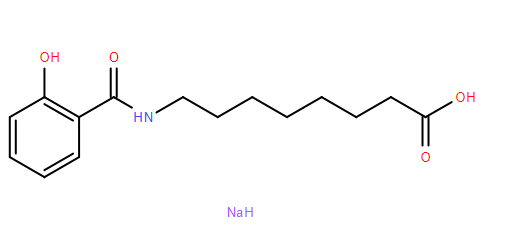
- Product Name:Salcaprozate Sodium
- Molecular Formula:C15H20NNaO4
- Purity:99%
- Molecular Weight:301.3134
Product Details:
CasNo: 203787-91-1
Molecular Formula: C15H20NNaO4
Factory Supply High Purity Salcaprozate Sodium 203787-91-1 Safe Transportation
- Molecular Formula:C15H20NNaO4
- Molecular Weight:301.3134
- Vapor Pressure:1.03E-11mmHg at 25°C
- Melting Point:183 - 185°C
- Boiling Point:521.7°Cat760mmHg
- Flash Point:269.3°C
- PSA:89.46000
- Density:g/cm3
- LogP:1.60340
Salcaprozate Sodium(Cas 203787-91-1) Usage
|
Description |
Salcaprozate sodium (SNAC) is the sodium salt form of salcaprozate, serving as an oral absorption promoter. It acts as a chemical permeation enhancer (PE) and is commonly utilized as a delivery agent to enhance the oral absorption of macromolecules, particularly those with poor bioavailability, such as insulin and heparin. In drug formulation, SNAC is employed as an excipient to aid the oral absorption of various macromolecules, peptides, and proteins, including insulin for diabetes, heparin for heart attacks and angina, and cyanocobalamin for vitamin B12 deficiency and anemia. |
|
Uses |
Salcaprozate sodium is considered safe for human consumption and has obtained Generally Recognized as Safe (GRAS) status from the FDA. It is known to be one of the most advanced chemical permeation enhancers, alongside sodium caprate (C10), and both are generally acknowledged as safe and efficacious for use in marketed drug products. Salcaprozate sodium, also referred to by its chemical name sodium 8-(2-hydroxybenzamido)octanoate, is supplied as a hygroscopic white to off-white solid by Actylis. |
InChI:InChI=1/C15H21NO4.Na/c17-13-9-6-5-8-12(13)15(20)16-11-7-3-1-2-4-10-14(18)19;/h5-6,8-9,17H,1-4,7,10-11H2,(H,16,20)(H,18,19);/q;+1/p-1
203787-91-1 Relevant articles
Subchronic Oral Toxicity of Salcaprozate Sodium (SNAC) in Sprague-Dawley and Wistar Rats
M. Gary I. Riley griley@emisphere.com, M. Cristina Castelli, and Ellen Angela Paehler
International Journal of Toxicology, Volume 28, Issue 4
The present report summarizes the findings of 2 separate studies that explored the subchronic oral toxicity of SNAC in Sprague-Dawley and Wistar rats. The first study, sponsored by Emisphere Technologies, Inc (Cedar Knolls, New Jersey), employed SNAC alone and in combination with heparin.
A head-to-head Caco-2 assay comparison of the mechanisms of action of the intestinal permeation enhancers: SNAC and sodium caprate (C10)
Caroline Twarog a b, Kai Liu b c, Peter J. O'Brien a, Kenneth A. Dawson b c, Elias Fattal d, Brigitte Illel e, David J. Brayden a b
European Journal of Pharmaceutics and Biopharmaceutics Volume 152, July 2020, Pages 95-107
The MCFA, sodium caprate (C10), and the C8 derivative, salcaprozate sodium (SNAC), are the key components of the most advanced solid-dose oral peptide formulations with over 20 year’s experience in clinical trials. SNAC (Emisphere, NJ, USA) has been assessed in multiple Phase III PIONEER trials in tablets with the Glucagon-like-1 peptide analogue, semaglutide..
Relevant Products
-
Nemorexant
CAS:1505484-82-1
-
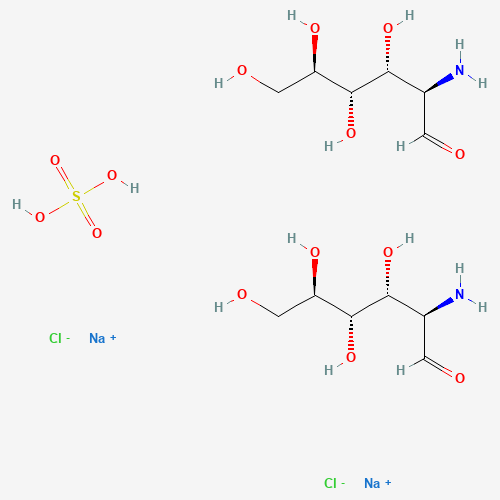
Glucosamine Sulfate Sodium Chloride
CAS:1296149-13-7
-
Riociguat
CAS:625115-55-1