606143-89-9
- Product Name:Binimetinib
- Molecular Formula:C17H15BrF2N4O3
- Purity:99%
- Molecular Weight:441.23
Product Details:
CasNo: 606143-89-9
Molecular Formula: C17H15BrF2N4O3
Purity: 99%
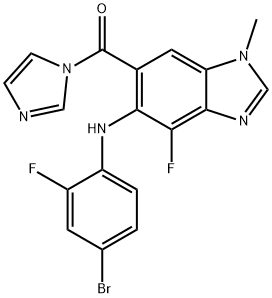
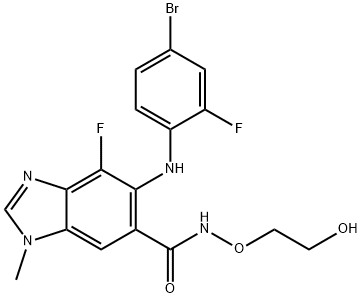
Synonyms: 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide; MEK162(ARRY-438162); MEK162(ARRY-162,ARRY-438162); BiniMetinib(MEK162,ARRY-162,ARRY-438162); 1H-Benzimidazole-6-carboxamide,5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-; 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamideBinimetinib(MEK162,ARRY-162,ARRY-438162); ARRY-4381625-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide
Melting point: >203°C (dec.)
Density: 1.67
Storage temp.: -20°C
Solubility: Soluble in DMSO (up to at least 25 mg/ml)
Form: solid
Pka: 14.20±0.10
Color: White
Stability: Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months.
Kinase inhibitor:
Binimetinib, also known as Mektovi, is a potent and selective oral mitogen-activated protein kinase 1/2 (MEK 1/2) inhibitor with potential antineoplastic activity.
Binimetinib, noncompetitive with ATP, binds to and inhibits the activity of MEK1/2. Inhibition of MEK1/2 prevents the activation of MEK1/2 dependent effector proteins and transcription factors, which may result in the inhibition of growth factor-mediated cell signaling. This may eventually lead to an inhibition of tumor cell proliferation and an inhibition in production of various inflammatory cytokines including interleukin-1, -6 and tumor necrosis factor.
Mechanism of Action:
Binimetinib is a reversible inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activity. MEK proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, binimetinib inhibited extracellular signal-related kinase (ERK) phosphorylation in cellfree assays as well as viability and MEK-dependent phosphorylation of BRAF-mutant human melanoma cell lines. Binimetinib also inhibited in vivo ERK phosphorylation and tumor growth in BRAF-mutant murine xenograft models.
Pharmacokinetics:
The primary metabolic pathway is glucuronidation with UGT1A1 contributing up to 61% of the binimetinib metabolism. Other pathways of binimetinib metabolism include N-dealkylation, amide hydrolysis, and loss of ethane-diol from the side chain. The active metabolite M3 produced by CYP1A2 and CYP2C19 represents 8.6% of the binimetinib exposure. Following a single oral dose of 45 mg radiolabeled binimetinib, approximately 60% of the circulating radioactivity AUC in plasma was attributable to binimetinib.
Uses:
MEK 162 is a MEK1/2 inhibitor allowing it to be a effective anti-cancer medication.
Definition:
ChEBI: Binimetinib is a member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-fluorophenyl)nitrilo, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectively. It is a MEK1 and MEK2 inhibitor (IC50= 12 nM). Approved by the FDA for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation in combination with encorafenib. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzimidazoles, a member of bromobenzenes, a member of monofluorobenzenes, a hydroxamic acid ester and a secondary amino compound.
Relevant Products
-
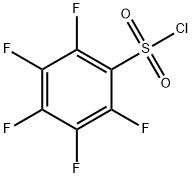
Pentafluorobenzenesulfonyl Chloride
CAS:832-53-1